Abeywardane, Asitha S Jian, Wen and Shi,
Jianzheng
Introduction
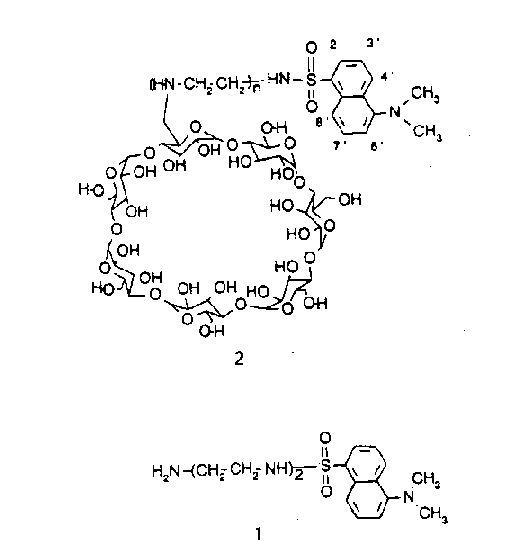
Beta-cyclodextrin is a cyclic oligosaccharide consisting of seven glucose units linked by beta-(1,4) glycosidic bonds [1].Beta-cyclodextrin forms inclusion compounds with smaller molecules like dansyl group (1), which fits into the 5-8 Angstrom cavity of cyclodextrin . Therefore, inclusion compounds consist of the "host" component (cyclodextrin) that admits the "guest" component (dansyl group) into its cavity without any covalent interactions . These cyclodextrin components exist in aqueous solutions . Therefore, they can be elegantly studied by NMR spectroscopy . NMR spectroscopy analysis reflects the atomic details in the complex in solution. Hence, with the aid of NMR spectroscopy one could identify the atomic interactions between the guest and the host. Further, this data can be used to establi sh the geometrical relationship between the guest and the host. In this paper the conformation of the dansyldietylenetriamine-modified beta-cyclodextrin (2) is studied by 2D-NMR spectroscopy [2]. That is, the general goal of this paper is to identify whet her the dansyl group is equatorial or axial to the cyclodextrin via 2D-NMR spectroscopy .

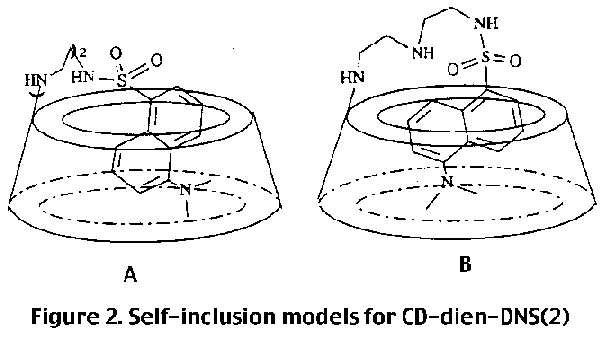
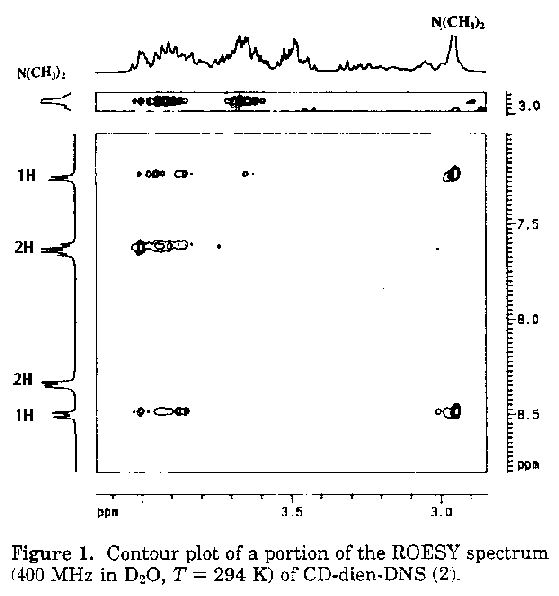
Since the problem deals with the relative orientation of cyclodextrin and dansyl groups, one needs to obtain through space 1H, 1H coupling. For this purpose ROESY (rotating frame overhauser enhancement spectroscopy) is used, instead of NOESY, in order to obtain overhauser effects from through space within 5 Angstroms, as, for cyclodextrin the former provides more reliable results than the latter [1] . The contour plots of portion of the ROESY spectrum showing connectivity of the aromatic and dimethyl amine protons with the signals in the aliphatic region is shown in figure 1. The connectivity through space with the protons of the dimethyl amino group allows the identification of the protons H4' and H6' of the naphthalene unit . Connectivities are observed between H3', H4', H6' and H7' and the cyclodextrin protons, while no NOE is observed in the same region for protons H2' and H8' of the naphthalene ring . This indicates that the protons H2' and H8' are outside the cyclodextrin ring, indicating that the molecule is equatorial.
Reference:
(1) Corradini, R.; Dossena, A.; Galaverna, G.;
Marchelli, R.; Panagia, A.; Sartor, G. J.Org.Chem. 1997, 62,
6283-6289.
(2) Two -Dimensional NMR Spectroscopy. Chapter 2, pp
337-43.; Croasmun, W. R., Ed.; VCH publishers: New York . 1994;
Spectrum
Questions
Question 1 (ICR)
Why was ROESY spectroscopy used instead of NOESY
spectroscopy in order to identify the conformation of
dansyldiethylenetriamine-modified beta-cyclodextrin ?
Question 2 (RQD)
Identify the chemical shifts of protons H4' and H6'?
Question 3 (RQD)
What changes in the ROESY spectrum would you observe if
the -N(CH3)2 is shifted from position "5" to position "6" ?
Question 4 (RQD)
Calculate the chemical shifts of the protons in the
sugar molecule ?
Question 5 (EVL)
According to the provided information, which model in
figure 2 correctly represents the structure of compound 1?